- Your cart is empty
- Continue Shopping
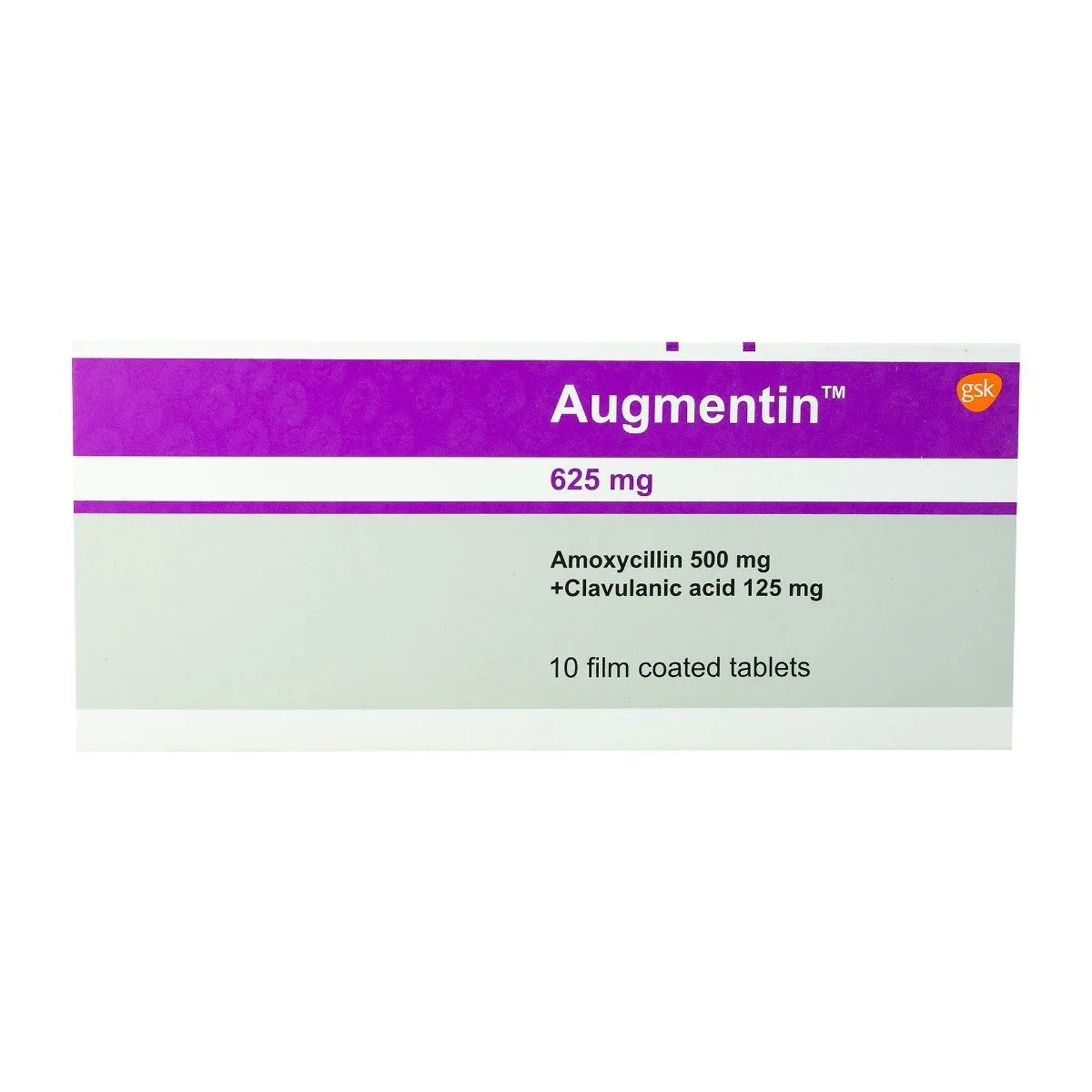
10 tablets
Medical Description
Antibiotic, belongs to the penicillin group used in the treatment of bacterial rhinosinusitis, pneumonia, otitis media, prophylaxis or treatment against bite wound infection, diabetic foot infection, odontogenic infection and urinary tract infection.
Indication & Usage
Warning: The doses below are based on the amoxicillin component only: -Usual dosing range: Oral: Immediate release: 500 mg every 8 to 12 hours or 875 mg every 12 hours; Extended release: 2 g every 12 hours. IV: 1 g every 8 hours or 2 g every 8 to 12 hours. -Acute bacterial rhinosinusitis: Standard dose: Oral: Immediate release: 500 mg every 8 hours or 875 mg every 12 hours for 5 to 7 days. High dose: Oral: Extended release: 2 g every 12 hours for 5 to 7 days. -Pneumonia: Oral: Immediate release: 875 mg twice daily. Extended release: 2 g twice daily. Duration of therapy: 5 days. -Urinary tract infection: In case of acute uncomplicated or acute simple cystitis: Oral: Immediate release: 500 mg twice daily for 5 to 7 days, while in case of complicated urinary tract infection: Oral: Immediate release: 875 mg twice daily for 10 to 14 days. -Peritonsillar cellulitis or abscess: Oral: Immediate release: 875 mg every 12 hours to complete a total of 14 days of therapy. -Acute otitis media: Oral: Immediate release: 875 mg twice daily or 500 mg every 8 hours. Extended release: 1 or 2 g twice daily. Duration: 5 to 7 days for mild to moderate infection and 10 days for severe infection. -Odontogenic infection: Oral: Immediate release: 875 mg twice daily; continue to complete a total of 7 to 14 days of therapy. -Intra-abdominal infection: Acute diverticulitis: Immediate release: Oral: 875 mg every 8 hours. Extended release: Oral: 2 g every 12 hours. Duration of treatment for diverticulitis or uncomplicated appendicitis managed without intervention is 7 to 10 days. -Diabetic foot infection: Oral: Immediate release: 875 mg every 12 hours. Duration of therapy should be tailored to individual clinical circumstances; most patients with infection limited to skin and soft tissue respond to 1 to 2 weeks of therapy. -For the rest of adult dosing: See dosage and administration.
Active Ingredients
Clavulanic Acid
Amoxicillin
Dosage & Administration
-Administer around-the-clock. -Administer with food to increase absorption and decrease stomach upset. Extended release tablets should be administered with food. -Adult dosing (continue): -Chronic obstructive pulmonary disease: Oral: Immediate release: 500 mg every 8 hours or 875 mg every 12 hours for 5 to 7 days. -Prophylaxis or treatment of bite wound infection (animal or human bite) for adults : Oral: Immediate release: 875 mg every 12 hours. For prophylaxis, duration is 3 to 5 days; for treatment of established infection, duration is typically 5 to 14 days and varies based on clinical response and patient-specific factors.
Side Effects
-Antibiotic-associated diarrhea (AAD), nausea, and vomiting, Clostridioides difficile infection (CDI), drug-induced liver injury, urticaria, angioedema and anaphylaxis. -Diaper rash, skin rash, urticaria, nausea, vomiting, vaginitis, candidiasis, vaginal mycosis, abdominal distress, flatulence, thrombocythemia and headache.
Safety Advice
-Should not be given to patients suffering from hypersensitivity to amoxicillin, clavulanic acid and other beta-lactam antibacterial drugs. -Use is contraindicated in patients with a history of amoxicillin and clavulanate-associated hepatic dysfunction. -In case of renal impairment: If the creatinine clearance is 10 to <30 mL/minute: Dose when needed: 250 to 500 mg every 12 hours, If the creatinine clearance is <10 mL/minute: 250 to 500 mg every 12 to 24 hours. -Prolonged use may result in fungal or bacterial superinfection. -In case of over dose be ready to tell or show what was taken, how much and when it happened, and seek immediate medical attention. For additional information call us on 16676. Always tell your physician your detailed medical history.
Storage
Store at room temperature.
Drug Interactions
-Allopurinol: May enhance the potential for allergic or hypersensitivity reactions to Amoxicillin. -Aminoglycosides: Penicillins may decrease the serum concentration of Aminoglycosides. -Acemetacin: May increase the serum concentration of Penicillins. -BCG Vaccine: Antibiotics may diminish the therapeutic effect of BCG Vaccine. -Methotrexate: Penicillins may increase the serum concentration of Methotrexate. -Mycophenolate: Penicillins may decrease serum concentrations of the active metabolite(s) of Mycophenolate. -Sodium Picosulfate: Antibiotics may diminish the therapeutic effect of Sodium Picosulfate. -Tetracyclines: May diminish the therapeutic effect of Penicillins. -Vitamin K Antagonists (eg, warfarin): Penicillins may enhance the anticoagulant effect of Vitamin K Antagonists.
Pregnancy & Lactation
-Both amoxicillin and clavulanic acid cross the placenta. An increased risk of necrotizing enterocolitis in neonates or bowel disorders in children may be associated with amoxicillin/clavulanate when exposure occurs near delivery, as a class, penicillin antibiotics are widely used in pregnant women. Based on available data, penicillin antibiotics are generally considered compatible for use during pregnancy, Amoxicillin/clavulanate is considered compatible for the of treatment airway diseases in pregnant women. Use should be avoided in women at risk for preterm delivery. -Amoxicillin is present in breast milk following administration amoxicillin/clavulanate. The relative infant dose (RID) of amoxicillin following administration of amoxicillin/clavulanate is 0.02% to 0.07%. In general, breastfeeding is considered acceptable when the RID is <10%, amoxicillin/clavulanate is considered compatible with breastfeeding when used in usual recommended doses. -Ask your physician before taking any medication during pregnancy or lactation.